Discovery Spotlight
Emery Bresnick, PhD
Gary Felsenfeld Professor
Department of Cell & Regenerative Biology
Hematopoiesis, Blood Cancer Mechanisms and Genome/Epigenome Function
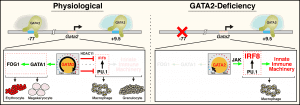
The Bresnick group conducts basic and translational research on genomics/epigenomics, hematopoiesis and myeloid/erythroid cell biology. We discovered GATA2 enhancers that are essential for hematopoiesis and embryonic development, and their disruption causes human diseases. Our studies unveiled paradigms to explain hematopoietic stem cell generation, progenitor fate decisions and erythrocyte development. Germline mutation of the +9.5 enhancer (or coding mutations) cause GATA2-deficiency syndrome, which involves immunodeficiency, bone marrow failure and predisposition to develop myelodysplastic syndromes and acute myeloid leukemia. An additional enhancer discovered by the Bresnick group (-77) is expropriated by the protooncogene EVI1, thus defining a novel leukemia mechanism. Clinical centers screen for genetic variation in these enhancers to diagnose the molecular basis of blood diseases and direct clinical decisions. We are expanding the mechanistic depth of this discovery science and conducting multi-disciplinary studies of pre-malignant states. We identified GATA2-instigated networks that control hematopoiesis and are deciphering the importance of components of these networks, including many genes/proteins representing potential targets to enhance the diagnosis, treatment and prevention of bone marrow failure, leukemia and clonal hematopoiesis of indeterminate potential (CH). CH afflicts large numbers of people, especially the elderly, and is associated with blood cancers and cardiovascular disease. The GATA2 work guided genomic studies that identified a large enhancer cohort predicted to serve critical roles. One of these enhancers controls expression of a new regulator of c-Kit receptor tyrosine kinase signaling, Samd14, which confers survival in anemia. Studies are testing whether dysregulation of these components in families with a history of hematologic disease, yet lack mutations in established hematopoietic regulators, corrupts GATA2 genetic networks as a pathogenic mechanism. Screening systems are being developed to rescue network defects to overcome differentiation blockades. We use multi-omic (quantitative proteomic, transcriptomic, metabolomic and lipidomic) approaches in cell populations and single cells to understand stem/progenitor cells as well as myeloid and erythroid cell biology. These studies have important fundamental and translational implications, including how the innate immune system and epigenetic mechanisms operate in normal and disease contexts. Of particular note are discoveries of epigenetic mechanisms controlling differentiation, small molecule determinants of genome/epigenome function, RNA exosome complex requirement to balance proliferation and differentiation and metal-dependent mechanisms that control survival and differentiation.
Publications:
https://www.ncbi.nlm.nih.gov/myncbi/1daRo5QftwzQb/bibliography/public/